The NLRP3 Inflammasome and Chronic Inflammation (Part 1)
The biological role of the NLRP3 inflammasome and its many triggers of activation
In this series of articles on inflammasomes, specificially NLRP3, I will outline its role in various health conditions, including problems linked to autism, as well as treatment options to potentially offset problems from NLRP3 overactivation. To gain a better appreciation for what inflammasomes are and how they behave, it’s important to first establish some foundational understanding of certain cells within the innate immune system.
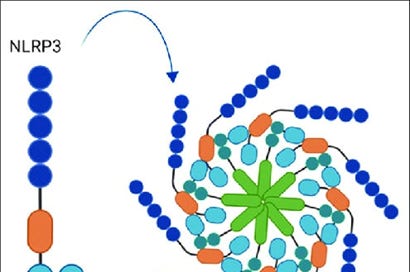
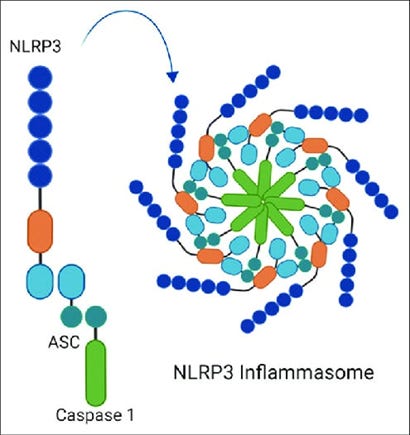
Image 1 - Basic structure of the NLRP3 Inflammasome containing the NLRP3 sensor, ASC adaptor, and the caspase 1 enzyme.
The NLRP3 inflammasome is a multi-protein complex of the innate immune system that plays a central role in detecting cellular stress and triggering inflammation. It is part of the NOD-like receptor (NLR) family and acts as a sensor for pathogen-associated molecular patterns (PAMPs) and damage (or danger) associated molecular patterns (DAMPs).
The innate immune system includes a variety of cell types such as neutrophils, eosinophils, dendritic and mast cells, monocytes, and macrophages. These cells are part of the first-line immune defense against pathogens and other antigens and communicate through chemical signaling and dentritic cell messaging with the adaptive immune system which include B-cells, T-cells, and Natural Killer Cells (NK cells…although NK cells are sometimes listed as innate immune cells).
Image 2 - Innate immune cells such as neutrophils, monocytes, and macrophages play a significant role in first line immune defense. The adaptive immune system such as T-cells are engaged in part through information being transferred to them from dendritic cells (DC). The DC is an immune cell bridge between the innate and adaptive immune system.
Pattern recognition receptors (PRRs) on innate immune cells (see image below) recognize various PAMPs associated with microbes which includes bacteria, fungus, viruses. The interaction of a PRR with a PAMP initiates an innate immune cell activation sequence for lysis of the organism and/or cytokine production which can involve inflammation.
Image 3 - There are soluble PRRs and membrane bound PRRs on innate immune cells. Each PRR is designed to recognize unique molecular configurations (aka molecular patterns) of a PAMP.
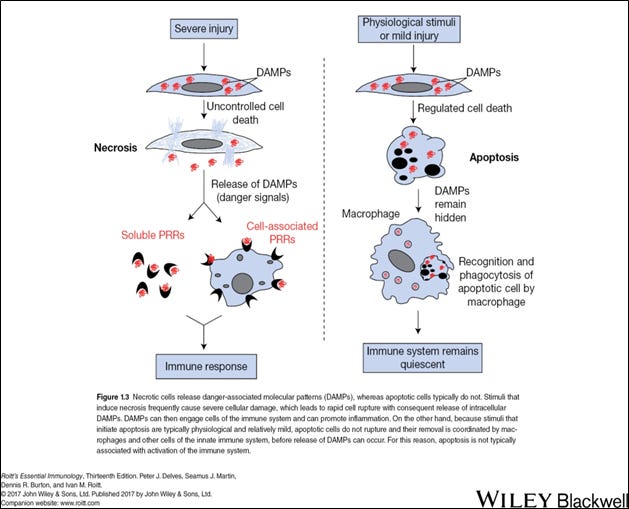
Damage or danger-associated molecule patterns (DAMPs) are associated with our own cellular structures (see image below). They include various components of cells from proteins, DNA material, and cell membranes. When a cell is mildly injured or has lost it usefulness a process of cellular removal is initiated called apoptosis. Apoptosis is a tightly controlled mechanism for the removal of “old and worn-out” or deranged cells without unmasking DAMPs. In this process, certain innate immune cells are activated such as a macrophage, but there is no trigger of damaging local or systemic inflammation, and the overall immune system remains relatively quiet. This is in contrast to severe cellular injury which could occur from trauma, chemical toxicity or massive infection where DAMPs are released in mass initiating cell-associated and soluble PRR recognition.
Image 4 - Danger (or damage) associated molecular patterns (DAMPs) are sequestered within a cell and not seen and recognized by PPRs of innate immune cells. It’s not until there is significant cellular injury or dysfunction where a DAMP interacts with a PRR and generates an immune response resulting in significant inflammation.
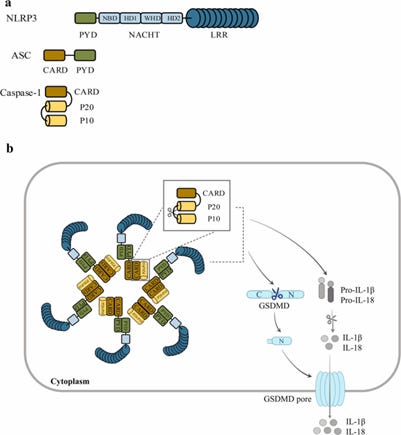
The structure of the NLRP3 inflammasome consists of three main components:
NLRP3 (NOD-, LRR-, and pyrin domain-containing protein 3) – The sensor protein that detects danger signals.
ASC (Apoptosis-associated speck-like protein containing a CARD) – An adaptor protein that links NLRP3 to caspase-1.
Caspase-1 – An enzyme that processes pro-inflammatory cytokines like IL-1β and IL-18, making them biologically active.
Image 5 - The main components of a typical NLRP3 inflammasome which contains a sensor (for various antigens), adaptor (which engages downstream enzyme activity), and the caspase enzyme itself. It’s the caspase-1 enzyme that acts to cleave pro-cytokines of Interleukin-1 beta (IL-1β) and Interferon-18 (IL-18) to become active pro-inflammatory cytokines.
The mechanism involved with NLRP3 inflammasome activation occurs from the presence of various antigens (a substance that induces an immune response) which come in many forms:
Pathogens such as bacteria, fungus, and viruses (e.g., RNA), including the spike protein of SARS-CoV2.
Endogenous danger signals such as ATP and uric acid crystals. There are many other signals too in this category worth noting such as amyloid, oxalic acid, components of mitochondrial damage, and cholesterol crystals.
Environmental irritants or toxins which include aluminum, asbestos, silica, etc.
Image 6 - The NLRP3 inflammasome complex is the most studied of the inflammasome subtypes and has multiple activators that occur through various cell membrane receptors or intracellular imbalances and organelle damage.
The process of NLRP3 activation and downstream events follows a predictable sequence:
NLRP3 recruits the ASC adaptor protein
ASC then binds to and activates caspase-1 enzyme
Caspase-1 cleaves both pro-IL-1β and pro-IL-18 into their active inflammatory forms.
Both IL-1β and IL-18 can induce pyroptosis which is an inflammatory cell death reaction.
The NLRP3 inflammasome is important for fighting infections, but its overactivation can lead to various diseases, including:
Autoimmune or inflammatory diseases such gout, multiple sclerosis, and rheumatoid arthritis.
Detrimental effects from infections such as lung damage during acute SARs-CoV-2 and bacterial sepsis.
Metabolic disorders which include Type I diabetes, atherosclerosis, and subsequent cardiovascular disease.
Neurodegenerative diseases such as Alzheimer' and Parkinson's
A recent article in Psychiatry Research (2024) discusses NLRP3 Inflammasome activation, IL-18 and IL-1β elevation, and other immune chemicals being linked to autism-spectrum disorder. This article and other information related NLRP3, including treatment options, will be explored in subsequent Substack articles in this series.