Viral Persistence in Long COVID
Mechanisms related to digestive system cells that may harbor SARS-CoV-2 virus or viral fragments
An excellent article by P. McMillan, et. al. published through Preprints.org in July 2024 provides compelling and concerning information regarding the potential for specific digestive cells as a medium for harboring SARS-CoV-2 virus or viral particles, including mRNA. The authors propose a hypothesis that “Long Covid (post-acute sequelae of COVID-19, PASC) is primarily a disease driven by the gut that is perturbed by underlying inflammation.”
What follows is an outline of key points I want to emphasize from this article. For a more detailed understanding of the various mechanisms linked to this discussion, including references, it is recommended to access and read the article in its entirety - HERE.
ACE-2 Receptor and Organ Systems
The ACE-2 receptor is widely distributed throughout the body, but heavily concencentrated in cells involving blood vessels, brain, heart, kidneys, lungs, testes, and intestines. This receptor is used by SARS-CoV-2 as its primary route of entry into cells, although other receptors are now being recognized.
In the nasopharyx and upper lungs, the virus infects cells through binding to ACE-2. Once infected, the virus uses various strategies to evade interferon (i.e., antiviral cytokine) responses from the host immune system which allows further infection and asymptomatic spread to the lower lungs.
Within the lungs, additional viral replication occurs along with alveolar macrophage activation causing inflammation. Local spread to lung endothelial cells allows for blood vessel penetration and dissemination throughout body, including intestinal cells.
Within the intestines there are many potential cellular targets for harboring virus. Intestinal biopsies have demonsrated concentrations of viral protein, including nucelocapsid and spike protein in specific regions of the intestines such as the duodenum, ileum, and colon. Even thouugh confirmation of cultured virus is not always established, biopsy specimens have contained viral mRNA. This is concerning as the mRNA injections for SARS-CoV-2 provide mRNA and its lipid nanoparticle component which is also known to circulate throughout the body.
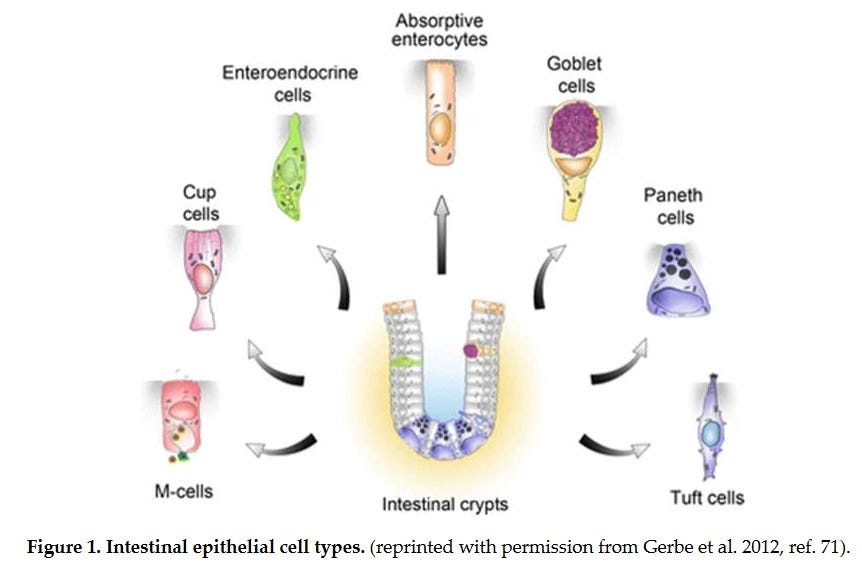
The following image (Figure 1) shows various cells within the digestive system. Some of these cells are suspected of harboring mRNA or other SARS-CoV-2 viral components. Absorptive enterocytes make up the bulk of cells of the digestive tract necessary for nutrient absorption. However, because of their rapid turnover (approximately 5 days) the authors feel these cells are not likely the main culprit for viral replication.
Image 1 - There are multiple cells that make up the intestinal lining. The main absorptive cell is enterocytes, and other cells are involved in everything from antimicrobial peptide production, mucus formation, and hormone regulation.
Main Cells of Concern
The following three cells are felt most likely to provide the potential of location for viral remants related to SARS-CoV-2 and drivers for persistent immune responses in Long Covid. Please note that these impressions come for the articles authors, but is not absolute confirmation that other cells too in the digestive system could play a role in viremia:
Enterochromaffin cells are neuroendocrine cells within the intestinal lining that secrete gut peptides which influence the intestines directly, as well as distant organs (e.g., brain). They comprise less than 1% of intestinal cells, but their control on gut inflammation and motility is important.
Tuft cells produce biogenic amines and peptides such as serotonin. They have the ability to sense the local environment within the digestive system. They are likely a target for ongoing infection and subsequent viremia and are known to evade immune clearance once compromised by virus.
Cup cells, like tuft cells, contain unique G-proteins receptors. A cup cell G-protein called vimentin is reported to be a co-receptor facilitating SARS-CoV-2 cell entry.
The concern regarding G-protein receptors beyond cup cell vimentin from is that some Long Covid symptoms are associated with auto-antibodies directed against these receptors. For example, some taste and smell responses are mediated by G-protein receptor activation.
Additional Information
Smell and taste abnormalities of tuft cells are likely the main driver for some Long Covid sensory symptoms.
Cup cell involvement, with its G-protein receptor as a target for autoantibodies, could lead to low cortisol seen in Long Covid, and associated fatigue. This G-protein involvement could also interrupt normal cortisol feedback activity within the pituitary. The pituitary has regulatory control over the adrenal cortex for cortisol production.
The infecion of enterochromaffin cells might cause postural orthostatic tachycardia syndrome (POTS), along with depression.
Prolonged fecal shedding or viral RNA has been documented up to 200+ days post infection and points to the intestines as the source of ongoing viremia.
The gut-brain axis is important to recognized too. Immune cell activity within the digestive system such as macrophages, monocytes, and mast cells can be related to systemic activity too, including the brain and nervous system. Various areas in the brain can be cellular targets for infection.
There are reports of other cells in the body serving as reservoirs for replicating virus, including the thalamus, hypothalamus and neurons. Dysfunction of these brain centers and neurons in general have huge implications for neurocognitive disorders, neurodegeneration, and other other brain problems such as brain fog and memory.