Mitochondria and the Electron Transport Chain (Part 5)
The unique function of complex II within the Krebs cycle and electron transport chain
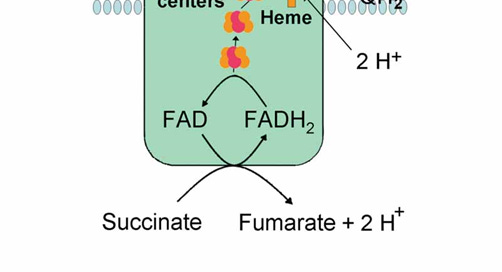
Image 1 - complex II of the electron transport chain is linked to the Krebs cycle through its conversion of succinate (succinic acid) to fumarate (fumaric acid). FAD (flavin adenine dinucleotide) is associated with vitamin B2, riboflavin.
Complex II (aka Succinate dehydrogenase) is the smallest complex of the electron transport chain (ETC), particularly compared to complex I. Like complex I, it has a significant portion of its structure within the matrix of the mitochondria (see image below), and contains only 4 protein subunits compared to the 46 of the first complex. There are some other similarities of complex II with complex I such as iron-sulfur clusters for electron transport, and dependence on the presence of vitamin B2 (riboflavin). However, there are some unique characteristics of complex II that don’t exist with any of the other protein complexes of the ETC.
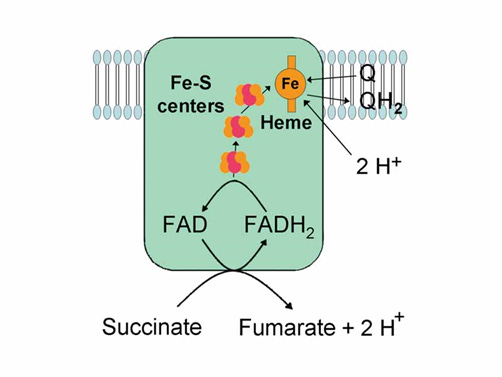
Image 2 - complex II sits between complex I and III and is involved in transporting electrons from complex II to complex III. Notice too it contains iron-sulfur (Fe-S) clusters.
Complex II (Succinate dehydrogenase, SDH or Succinate-coenzyme Q reductase, SQR)
Complex II is uniquely positioned within the ETC for the transfer of electrons to complex III. It’s basic structure and function, includes:
SDH is involved in the both the ETC and Krebs cycle (Kc).
Consists of 4 protein subunits and 3 iron-sulfer (Fe-S) clusters.
Does not pump protons (H⁺) from the matrix into the intermembrane space (IMS).
Transfers electrons (e⁻) from succinate to CoQ₁₀ via FADH₂.
Other electron donors such as fatty acids enter the ETC at complex II via FADH₂.
Another important aspect of complex II is that it’s entirely encoded by nuclear DNA. Mitochondrial DNA (mtDNA) and subsequent ribosome produced proteins are not part of the structural subunits of complex II. For more information about mitochondrial genetics see part 3 of this article series. Isolated complex II deficiency accounts for approximately 2% of all mitochondrial diseases.
Image 3 - The mitochondrial genome contains 22 transfer RNA-encoding genes and 13 protein-encoding regions. The 13 encoding regions are highly specific for critical aspects of electron transport chain. Notice that complex II is not represented in the genome of the mitochondria.
Krebs Cycle and Electron Transport Chain Activity
Complex II is the only complex of the ETC that functions within the Krebs cycle too. The Succinic acid dehydrogenase (SDH) enzyme converts succinic acid to fumaric acid (Krebs cycle reaction). This is important because total production of nicotinamide adenine dinucleotide (NADH), for activation of complex I and subsequent production of adenosine triphosphate (ATP), is partly dependent on SDH activity. This information is discussed in article 4 of this series.
Another important component of complex II is its iron-sulfur (Fe-S) clusters involved in electron transfer. These clusters are vulnerable to oxidative stress which is a natural reaction occurring within all mitochondria to some degree. Too much oxygen radicals, aka reactive oxygen species, is damaging to the ETC protein complexes, including complex II. Keep in mind that complex II sits between complex I and III which are the two greatest areas of electron leakage within a mitochondrion. Also, the bulk of its structure is within the mitochondria matrix where molecular oxygen resides and leakage of electrons from complex I and III can generate high levels of ROS that adversely effect complex II. Notice in the image below that only a small portion of complex II is within the inner mitochondrial membrane (IMM).
Image 4 - complex II contains 4 protein subunits, multiple iron-sulfur clusters (3 in total), and two unique subunits (SDHC and SDHD) which are embedded within the inner mitochondrial membrane and involved in electron transfer from the Fe-S clusters and the conversion of ubiquinone to ubiquinol.
Oxidative Stress, Free Radicals, Toxins, and Complex II Dysfunction
Interference with complex II can occur from many factors:
High levels of ROS damaging protein subunit function.
Oxygen or other radical formation compromising Fe-S cluster activity leading to poor electron transfer.
Inhibition of Succinic acid dehydrogenase conversion of succinate to fumarate.
Vitamin B2 (Riboflavin) deficiency.
Coenzyme Q₁₀ deficiency.
Genetic mutations affecting one or more of the 4 protein subunits or the various assembly factors that maintain the structural integrity of complex II.
Damage of the IMM housing the SDHC and SDHD.
Environmental toxins compromising complex II activity. For example, glyphosate is reported to compromise complex II function.
A significant problem with poor antioxidant capacity within the mitochondria, i.e., deficiency of glutathione, is the vulnerability of Fe-S cluster function. These clusters undergo one electron transfer reactions which which require a change in their positional orientation within the protein complexes (I, II, and III). The following image shows a conformation change in an iron-sulfur complex. A consistent redox status needs to be maintained in order for the functional orientation to occur for ongoing electron transfer.
Image 5 - the Fe-S clusters undergo contraction and relaxation as part of their structural transformation involved in one-electron transfer.
These Fe-S clusters also contain cysteine residues which through their thiol (-SH) component are vulnerable to adverse chemical reactions or attack by various substances, including heavy metals.
One example of poor antioxidant capacity and compromised Fe-S cluster function occurs within the Krebs cycle and the aconitase enzyme. Aconitase converts citrate (citric acid) into isocitric acid. However, in the presence of a mitochondrial glutathione the aconitase enzyme is compromised because of faulty Fe-S cluster positioning (see image below).
Image 6 - aconitase converts citrate to isocitrate within the Krebs cycle. It’s vulnerable to malfunction if the iron-sulfur clusters are compromised from genetic mutations or excessive oxidative stress, and antioxidant deficiency.
This has detrimental effects for downstream Krebs cycle activity as two immediate subsequent conversion reactions produce two of three NADH compounds needed to fully engage complex I of the ETC.
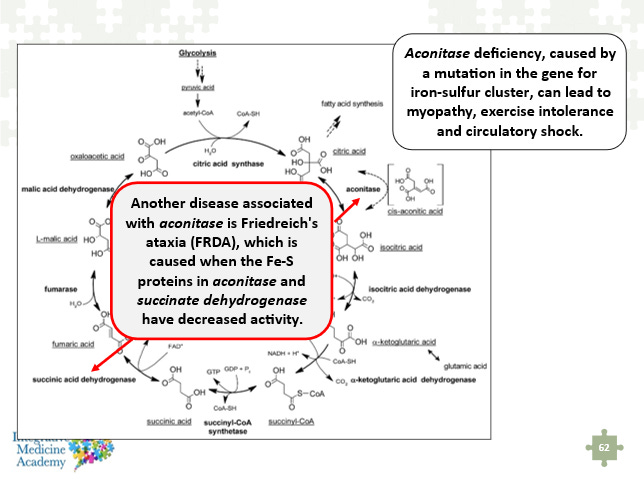
An important biochemical connection to understand with this discussion of iron-sulfur clusters, oxidative stress, etc. is that the succinic acid dehydrogenase enzyme also contains these same clusters. The following image is taken from my Mitochondria Mastery Course and shows aconitase deficiency from a mutation in the gene for iron-sulfur clusters, and the potential impact on SDH. Friedreich’s ataxia is a devastating genetic disorder that leads to progressive loss of muscular and cardiovascular function. This is discussed in part 1 of this article series.
Image 7 - this image shows the relationship between the aconitase and succinic acid dehydrogenase enzymes and their iron-sulfur clusters.
Isolated Complex II Deficiency Disease
The pathogenic variants affecting complex II genes results in mitochondrial dysfunction. From a genetic standpoint isolated complex II deficiency is a rare inborn error of metabolism, accounting for approximately 2% of mitochondrial disease diagnoses. The clinical presentation is similar to other mitochondrial diseases such as Leigh syndrome, often occurring during infancy and in many cases having catastrophic health affects. For an overview of clinical manifestations of many mitochondrial diseases read article 3 of this series.
A unique and unfortunate consequence of complex II deficiency linked to certain somatic mutations is susceptibility to tumorigenesis. For example, pathogenic variants in the SDHB and SDHC proteins have been associated with paragangliomas and pheochromocytomas. Other cancers, including gastrointestinal stromal tumors, pituitary adenomas, and renal cell carcinomas are recognized as well. For a more detailed discussion about this topic I recommend the following article from M. Fullerton, et al. titled “The genetic basis of isolated mitochondrial complex II deficiency.”
Conclusion
Complex II is critical for overall mitochondrial function both within the Krebs cycle and electron transport chain. Because of its position sitting between complex I and complex III it is vulnerable to damage from excess oxidative stress. It doesn’t pump hydrogen ions which become part of the electrochemical gradient for ATP production, but it is essential for transporting electrons from flavoproteins and the conversion of oxidized ubiquinone to reduced ubiquinol. All of this is an integral part of the flow of electrons necessary for conversion of molecular oxygen to water at complex IV.
This complex, like the others, is vulnerable to damage from oxidative stress and environmental toxins. Because of its relationship to both the Krebs cycle and electron transport chain damage of this complex could have very negative consequences on total mitochondrial activity. From a therapeutic standpoint, most of the literature focuses on reducing oxidative stress and supplementing with riboflavin as the main form of treatment. It’s likely that other complementary interventions could be helpful too such as those discussed with regards to complex I. The following short list of items are things to consider:
Vitamin B2 - 50mg/day (neonates), and 100mg to 300mg/day (adults).
CoQ10.
PQQ (pyrroloquinolone quinone) - check out my Substack article on PQQ and mitochondria.
MitoKatlyst - (-)-epicatechin.
Various antioxidants such as Sulforaphane and Ergothioneine - check out my Substack article on Longevity Vitamins.
Another therapeutic consideration for overall ETC support, particularly since complexes I, II, and III contain iron-sulfur clusters which need to be consistently supported in their redox cycleing is the use of molecular hydrogen. This will be a topic for a later Substack article, but for now I encourage to read an article from Joseph Mercola, DO.